In order to move forward letti needs to be acreative bopinionated - 6110574. And justify your answer.

Chemical Bonding Doodle Notes Science Doodle Notes Doodle Notes Science Doodle Notes Science Doodles
Chemical Bonding MUltiple Choice II Name.

. Both of these properties reflect the strength of the coulombic force. Lettie is having a problem in her experiment that she does not know how to solve. Formula units that contain two ions must.
Identify which ionic solid will have the higher melting temperature. The student can draw andor interpret representations of solutions that show the interactions between the solute and solvent. Draw representations of both ionic solids.
Both ionic of representations. Draw representations of both ionic solids The specific heats and molar heat capacity of a substance depend on both the molecular structure and intermolecular interactions for solids. And justify your answer.
The two sets of ions carry the. Draw charges on ion but not on water. The Sodium Chloride Structure 2.
H of the following isan ionic compound. A large number of ionic solids exhibit one of these five structures which are discussed here. Identity which ionic solid will have the higher melting temperature and justify your answer.
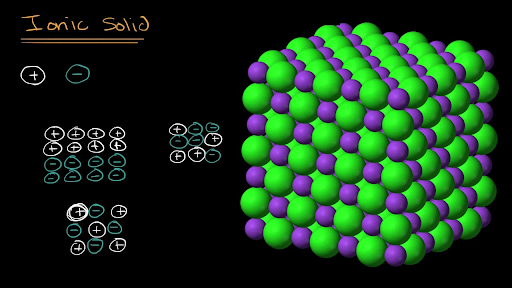
Hardness measures resistance to deformation. 1 Draw representations of both ionic solids in each set that show the relative. 1 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion.
Identify which ionic solid will have the higher melting temperature. Some Properties of Ionic Solids. In order to move forward letti needs to be acreative bopinionated - 6110574.
Because the ions are tightly bound to their oppositely-charged neighbors and a mechanical force exerted on one part of the solid is. The Zinc Blende Structure 3. Learn How to Draw Step by Step in a Fun wayCome join and follow us to learn how Written By leopoldohagos59926 March 26 2022 Add Comment Edit.
Identify which ionic solid will have the higher melting temperature. According to Coulombs Law these forces of attraction lattice energy increase as the distance between ionic centres decrease and as the charges on the ions increase. Draw representations of both ionic solids in each set that show the relative differences in size of the four ions the ratio of cations to anions and the charge on each ion.
Formula units that contain two ions must be drawn as networks consisting of twelve. Draw at least 3 water molecules around each. To deduce the type of bonding in a sample of a solid.
1 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. The structures which these solids adopt can be described in terms of the large anionscations occupying one or the other type of interstitial sites. The negative dipole oxygen side points toward cation and the positive dipoles H side points towards the anion.
7 Draw representations of both ionic solids in each set that show the relative differences in the sizes of the four ions the ratio of cations to anions and the charge on each ion. Draw representations of both solids that show the relative differences in the sizes of the four particles and the ratio of. See SP 11 LO 224 The student is able to explain a representation that connects properties of an ionic solid.
See SP 42 LO 223 The student can create a representation of an ionic solid that shows essential characteristics of the structure and interactions present in the substance. NaCl has a higher melting point because it has a lower atomic radius. Formula units that contain two ions must be drawn as networks consisting.
Powered by Blogger. MgBr2 and CaI2 are both ionic solids thus they both have very high melting temperatures due to the strong forces of attraction between ions. Ionic solids are composed of cations and anions held together by electrostatic forces.
Formula units that contain two ions must be drawn as networks consisting. Law a higher distance between ionic. As noted above ionic solids are generally hard and brittle.
And justify your answer. The Wurtzite Structure 4. Due to the strength of these interactions ionic solids tend to be hard brittle and have high melting points.
Ionic solids are poor conductors of electricity except when their ions are mobile such as when a solid is melted or dissolved in solution. ACh BN02 COF2 DSCI2 E cot k.

2 2 Docx Molecular And Ionic Compound Structure And Properties 2 2 Intramolecular Forces And Potential Energy Worksheet 1 Draw Representations Of Course Hero

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Ionic And Molecular Compounds Infographic Misses Out Giant Covalent Yet Lists Sio2 As Covalent Examp Chemistry Classroom Teaching Chemistry Chemistry Education
Representing Ionic Solids Using Particulate Models Video Khan Academy

Solved 7 Draw Representations Of Both Ionic Solids In Each Chegg Com

Learn The Difference Between Ionic And Covalent Bonds See Examples Of The Two Types Of Ch Ionic And Covalent Bonds Covalent Bonding Covalent Bonding Worksheet

How To Represent Ionic Solids Using Particulate Models Chemistry Study Com

0 comments
Post a Comment